Never before have we seen such severe difficulty in supplying raw materials, resulting in significant price increases.
Therefore, creating a safe and fast procedure to validate alternative raw material sources becomes essential so that we have different supply chains and can keep costs down.

Here we will look at how laboratories should deal with an alternative supplier validation request from their purchasing department, so it happens as quickly and economically as possible.
Just checking the INCI name alone could prove a mistaken, if not disastrous, choice. INCI names are often generic names covering materials whose formulations behave very differently. This is the case, for example, with polymers or raw materials of natural origin.
Irrespective of the current crisis, there are several reasons why we might want to approve alternatives.
- Reduced costs: having several potential suppliers for a raw material gives us greater negotiating power when it comes to prices.
- Shortages: The advent and spread of Coronavirus, climate change, and various choices in international trade are putting a strain on many companies’ operations and productivity. Even the best-known, historic manufacturers have been unable to meet the market’s demands, sometimes lengthening delivery times to an unsustainable extent.
- Improved warehouse management: Lower MOQs allow optimised warehouse capacity management and product shelf life, especially for low-turnover raw materials.
- Risk reduction: it is important not to depend on one supplier, especially for strategic raw materials. Several factors could reduce, even block, a supplier’s delivery of raw materials. Also, suppliers tend to fulfill the orders of their larger customers first, leaving smaller customers in the lurch.
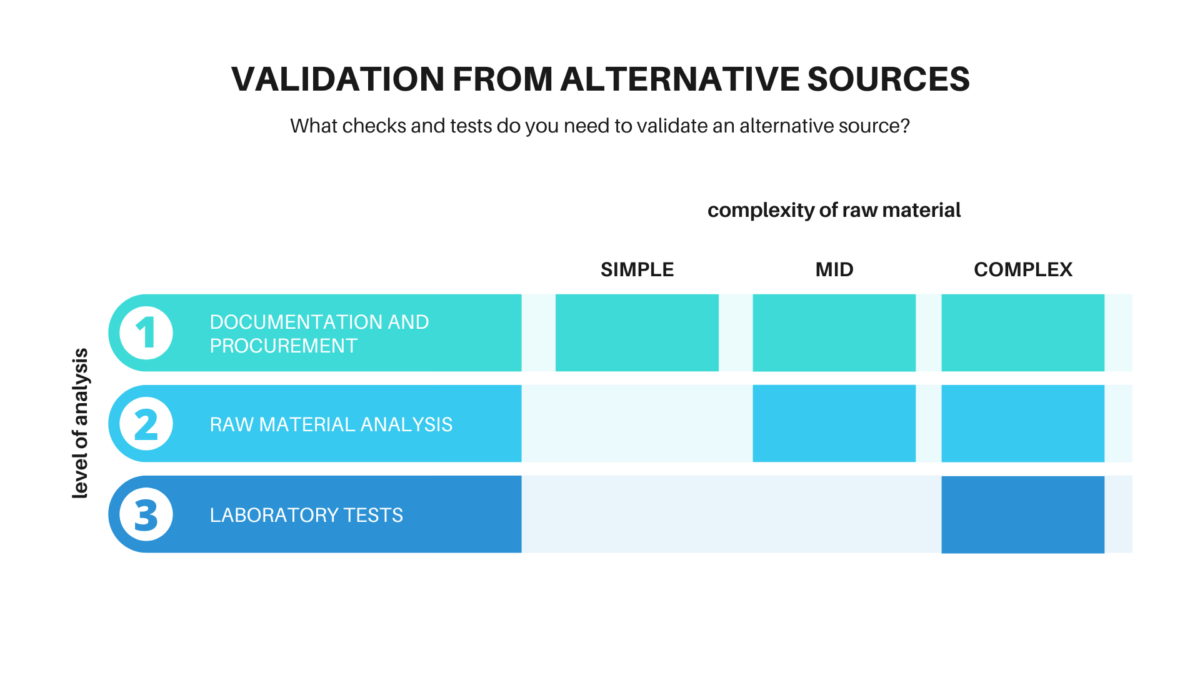
What checks and tests do you need to validate an alternative source?
1. DOCUMENTATION AND PROCUREMENT
First of all, you need to check the INCI composition and CAS numbers, including additives (preservatives, pH adjusters, …). The same ingredient may have different CAS numbers, so ask your supplier before rejecting it. Also, ask if the same raw material is available with other additives if you are not satisfied with the standard ones.
Analyse the specifications: unless you are a highly strategic customer for the manufacturer, specifications are non-negotiable. If there are only slight differences from your reference raw material’s specifications, try to analyse each parameter critically to understand if and how it might impact your finished product. You will be amazed at how irrelevant some parameters are.
Check compliance with regulations and directives relevant to you: raw materials for cosmetics, pharmaceuticals, food, ecolabels.
Make sure that your purchasing department has checked the MOQ, price and availability of the alternative raw material with the potential new supplier and enquired about the possibility of receiving samples.
For simple raw materials, with no composition variables such as glycerine, butylene glycol, cyclopentasiloxane or certain esters, nothing else needs to be done: you can just check the documentation.
2. RAW MATERIAL ANALYSIS
For products such as oils or plant extracts and esters, in addition to documentation, we need to check samples of the raw material for parameters that could impact the sensory qualities of the finished product: appearance, colour, and odour. Neither can we forget the taste if the ingredient is used in lip products.
In case of doubt, we can organise a simple triangular test involving laboratory technicians and marketing colleagues.
If the raw material comes in a different form, it is always best to discuss it with the production operators; for example, a wax that changes from bead to plate could complicate processing.
3. LABORATORY TESTS
Laboratory tests are necessary for more complex ingredients such as polymers, rheology modifiers, and solubilisers. To ensure that the functioning of the raw material to be validated is comparable to that of your standard, you will have to make a laboratory batch using the alternative in the company’s portfolio formula that contains it in the highest percentage. In addition to verifying the organoleptic qualities, you also need to analyse the chemical-physical parameters of the finished product, particularly pH and viscosity.
Laboratory tests are also recommended for formulas with lower percentages of the ingredient to be substituted but which are known to be more delicate, e.g. substitution of an emulsifier in a formula that is not perfectly stable at high temperatures, or solubiliser substitution in a solution that is not completely transparent at room temperature.
In the case of viscosity variations, it is essential to test the product in its final packaging to ensure no negative impact on its functionality, especially where a dispenser is used. Even the colour is best assessed in the final packaging because changes visible in large quantities may be invisible when the product is packaged for sale.
If the ingredient is critical to the product’s stability (e.g. emulsifiers), accelerated aging tests should also be carried out, possibly reducing the duration from 6-4 months to 3-1.
If an active ingredient is to be substituted, you must also consider the need to repeat any efficacy tests in addition to stability tests. This holds, for example, in cases of non-titrated plant extracts, which are obviously present in percentages that have to do with more than just marketing.
Examples of raw materials from alternative sources already successfully validated using the above scheme:
- MP 029672 DIBUTYL ADIPATE
- MP 030835 DICAPRYLYL CARBONATE
- MP 070008 DICAPRYLYL ETHER
- MP 025498 CETEARYL ISONONANOATE
- MP 030459 GLYCERYL MONOSTEARATE VEGETAL MB
- MP 020032 CETYL ALCOHOL
- MP 012003 MYRISTIC ALCOHOL 1498
- MP 012002 CETYLSTEARYLALCOHOL 50-50
- MP 034441 LAURYL GLUCOSIDE MB
- MP 034445 DECYL GLUCOSIDE MB
- MP 001708 XANTHAN GUM 200 MESH
- MP 010025 PVP VA 64 POWDER
- MP 010026 PVP K90 POWDER
- MP 010028 PVP K30 POWDER
- MP 001292 SILICON DIOXIDE 230
ECSA has been in the commodities trading market for over 100 years and has three warehouses with large storage capacities and wide availability of products for prompt delivery.
Thanks to its extensive network of suppliers and its strategy of not tying itself to a few specific manufacturers, ECSA can find the best alternatives on the market in terms of quality and price based on the customer’s specifications, regardless of the quantity required.
Contact our experts to ensure the continuity of your production!